SIRENS Projects
The projects in the SIRENS portfolio is presented in the following. Please contact us if you're interested in more information.

SIRENS Projects:
Companies need insight, input and testing in the development phase, from patients, clinicians and different hospital expertise. The aim of this project is to set up a professional system to facilitate feasibility and usability testing for different products and software. The purpose is to get faster and better support for companies in their development.
Stavanger university hospital collaborates with Helse Førde HF, Municipality of Kinn and Airlift Solutions AS to apply for the Norwegian Research Council's (NFR) "Health Pilot"-call. The consortium is given 0,3mNOK from NFR to develop such an application.
SIRENS Stroke Projects:
Educational and training materials are under development, tailored to the training programmes. The aim is to develop interactive clinical changing electronic teaching courses for each specialty participating in simulation-based team training.
Collaborating partners:
- Laerdal Medical AS
- Gordon Center for Simulation and Innovation in Medical Education (GCRME)
- Stavanger Acute Medicine Foundation for Education and Research (SAFER)

This project started as a quality improvement project at Stavanger University hospital aiming to reduce the time to treatment in patients with acute stroke. Efficient standard operating procedure, efficient team coordination, and communication is vital to keep treatment times as low as possible. We used in-situ simulation-based team training as a key part of our quality improvement project. A key part of simulation-based training in addition to recreating scenarios is debriefing the scenario. So far, the door-to-needle time has reduced from 30 to 13 minutes.
Collaboration partners:
- LHL Hjerneslag ung
- Laerdal Medical AS
- Sørlandet sykehus HF
- Akershus University Hospital
- Oslo University Hospital
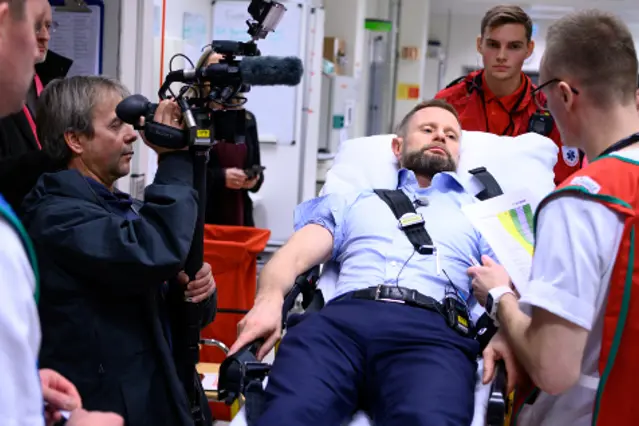
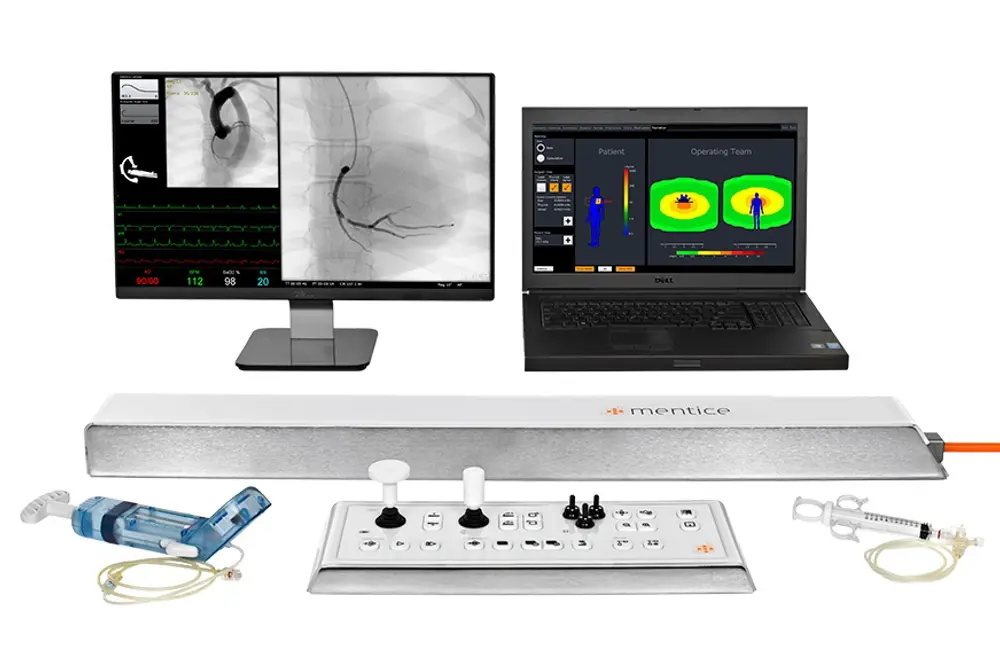
A technical skill set training is being developed for interventional radiologists at Stavanger University Hospital. The simulator is a portable, virtual reality simulator (Mentice VIST® G5 Simulator) including a wide variety of training scenarios. The technical reopening rate, the intervention time, and the clinical outcome of patients treated in the clinical setting are being monitored. Main focus of the cost-effective analyses will be how incurring costs through simulation training are mirrored by savings due to better patient outcome, less occurring complications, shorter hospital stay and generally reduced morbidity and mortality.
Collaborating partners:
- Sørlandet sykehus HF
- Akershus University Hospital
- Mentice AB
A multicentre technology development project on suspected stroke patients which will use microwave technology to differentiate hemorrhage from infarction in the acute phase. The measurement data will be evaluated for the presence of signal artifacts. The overall aim of this project is to evaluate the usability and develop diagnostic ability of Strokefinder MD100 to differentiate patients with hemorrhagic stroke (HS) and ischemic stroke (IS) /stroke mimics (SM) in the acute phase. The measurement data from patients will be used to generate a mathematical classification algorithm.
Collaborating partners:
- Helse Vest RHF (sponsor)
- Equinor ASA
- Medfield Diagnostics AB (co-sponsor)
- Haukeland University Hospital

Use facial recognition to detect early warning signes of stroke. There are two main different facial palsys: peripheral facial palsy and central facial palsy. The latter is tightly linked to stroke. By using mobile technology we plan to develop an algorithm able to detect central facial palsy.
Collaborating partners:
- ResQ Biometrics AS
- SimulaMet
- LHL Hjerneslag

Education of specialist registrars contains both practical and theoretical services. The education is based on learning objectives. A learning objective includes what the doctor shall understand or be able to carry out.
The learning objectives for specialist registrars, introduced 1st of March 2020, is regulated by The Norwegian Directorate of Health, while the responsibility to educate is the responsibility of the regional health authorities.
We are developing a Virtual Reality (VR)-based learning platform, in collaboration with Bouvet Norge AS. The platform uses simulation-based training related to the learning objective, as well as a tool for quality assurance.
Collaborating partners:
- Bouvet Norge AS

Stavanger university hospital has, together with the Municipality of Stavanger, LHL hjerneslag ung Rogaland, and Youwell AS, been funded 0,3mNOK to provide an application for a Norwegian Research Council's "Health Pilot"-call. The project aims to develop a more patient-controlled service, to significant improve experience and outcome measures.
This study is a Phase 3, randomized, multicentre, blinded, placebo-controlled, parallel group, single-dose, adaptive design with a single interim analysis for unblinded sample size re-estimation. Because AIS (acute ischemic stroke) is a medical emergency, the trial is designed to enable the administration of standard-of-care treatments without delay in order to save the life of the person concerned, restore good health or alleviate suffering.
Participants harboring an acute ischemic stroke who are selected for endovascular revascularization without intravenous or intra-arterial thrombolytic therapy will be given a single, 2.6 mg/kg (up to a maximum dose of 270 mg) intravenous dose of nerinetide or placebo. Outcomes of the main trial will be evaluated throughout a 90 day observation period.
The AXIOMATIC SSP study is a global, phase 2, randomized, double-blind, placebo-controlled, dose-ranging Study of BMS-987177, an oral factor XIa inhibitor, for the prevention of new ischemic stroke or new silent brain infraction in patients receiving aspirin and clopidogrel following acute non-hemorrhagic stroke or transient ischemia attack (TIA).
The purpose of this clinical study is to determine whether the addition of an oral Factor XIa Inhibitor to aspirin and clopidogrel is more effective than standard therapy in secondary stroke prevention.
- Bristol-Myer Squibb (sponsor)
- PPD Inc
